CLINICAL RESEARCH OFFICE Co., Ltd.
Our Experiences
Disease Areas
Malignant tumor
Neurosurgery
Diabetes
Gynecology
Orthopedics
Protocol
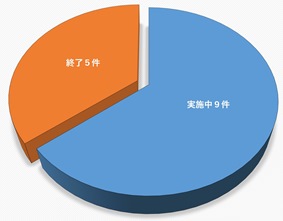
14 Protocols (5 closed, 9 ongoing)
Clinical Research
Investigators Initiated Clinical Trials (Post-Marketing)
Build SOPs
Prepare Case Report Form
Transcription for Case report form
Manual check
EDC query issue
Monitoring
Б@Б@Б@-5 trials ongoing (National University Hospital, Prefectural hospitals, Public hospitals, Corporate hospitals, Clinics, etc., 26 sites in total)
Б@Б@Б@Б@Disease Area: Endocrinology
Б@Б@Б@Audit (GCP, ICH-GCP)
Б@Б@Б@Б@-4 Protocols
Б@Б@Б@Б@Disease Area: Endocrinology, Ophthalmology, Gynecology
PhaseЗT/ЗU trial
Investigators initiated clinical trial
Б@Б@Б@1. PhaseЗT / ЗU anti-cancer agent
Б@Б@Б@2. PhaseЗT / ЗU anti-cancer agent
Phase III trial
Project management (medical equipment / extracorporeal shock wave)
Investigators initiated clinical trial (Medical device/ Neurosurgical program)
Б@Б@Б@Site Coordination Office
Б@Б@Б@Monitoring
Б@Б@Б@Registration/Allocation
Б@Б@Б@DM/ST
CLINICAL RESEARCH OFFICE Co., Ltd.
5-23-8 Kotsubo, Zushi-shi, Kanagawa, Japan 249-0008TEL +81 467-24-3209
FAX +81 467-24-3209